Chemical Compounds and the Environment
DO KIDS EVEN PLAY WITH LEGOS ANYMORE?
Recall that elements form everything in the universe, EVERYTHING!
Like LEGO, elements combine to form ionic and molecular compounds
Ionic and molecular compounds further combine, mix, and build upon each other to form literally everything.
Within this unit think of every object you see as a combination of chemical compounds stacked together like legos to form structures:
A wooden chair is made up of many many many molecules of C₆H₁₀O₅ (Cellulose)
Concrete is made up of many many many molecules of Ca₂SiO₄ (Calcium silicate)
DNA is made up of C₁₅H₃₁N₃O1₃P₂ (Deoxyribonucleic acid)
AN EXAMPLE OF HOW CELLULOSE THREADS (WHICH IS A CHEMICAL COMPOUND, DUH) COMBINE TO FORM THREADS THAT COMBINE TO FORM A TINY ROPE THAT COMBINES TO FORM A FIBER WHICH COMBINES WITH OTHER FIBRE TO CREATE THE CELL WALL, WHICH IS BASICALLY WOOD, TADAH!
THIS ISN’T THE SIDE OF A BASKET, THIS IS SIDE OF A CELL WALL. TAKE A TINY LITTLE ITTY-BITTY PLANT CELL, ZOOM IN ON ITS CELL WALL AND IT LOOKS LIKE INTER WEAVED THREADS
Naturally in our environment an infinite number of chemical reactions occur every second. Elements/ions will be ‘swapped‘ back and forth like a big game of uno. Natural processes constantly swap elements, back and forth, multiple times! This swapping forms a cycle. For instance look at:
photosynthesis - converts carbon dioxide and water into sugars
cellular respiration - converts sugars into carbon dioxide and water
forest fires (combustion) - converts cellulose into carbon dioxide and water
Long-story-short, life is basically minecraft just with chemical compounds as building blocks, and any structure imaginable is made by stacking chemical compounds together in a certain pattern.
How chemical compounds move in our environment
Soot (C₃H) in the atmosphere from the 1980 eruption of mount St. Helens spread as far as Edmonton. The photo of the gentleman shovelling soot off the roof was taken in Washington State shortly after the eruption.
Understand that chemical compounds spread around our planet/environments via:
air/atmosphere
soil/groundwater
surface waters (oceans, lakes, rivers)
Also from here on in I will mostly be referring to chemical compounds as “chemicals”
TRANSPORT OF CHEMICAL compounds IN AIR
When a substance is dumped into the atmosphere it’s considered to be airborne. The chemical will scatter.
Airborne chemicals will scatter in the atmosphere, how far they scatter depends on a few factors:
1. Source - Release of the chemical from the source, where a chemical is dispersed in the atmosphere
2. Chemical’s characteristics - weight, size, and surface area of chemical compound (over time airborne chemicals will settle and land on soil or water. The time to settle could be minutes - years.
3. Precipitation - rain/snow/humidity can all wash out airborne chemicals (however it deposits the chemical on the ground)
4. Wind - wind’s speed and direction (N,E,S,W)
Remember S-C-P-W
A HEAT-MAP OF NO₂ GAS IN ALBERTA AIR, THE LITTLE MOVING TICKS INDICATE WIND MOVEMENT. NOTICE MOST OF THE GAS MOVES TOWARDS THE SOUTH EAST REGIONS OF THE MAP, WHY?
A HEAT-MAP OF NO₂ GAS IN ALBERTA AIR, THE LITTLE MOVING TICKS INDICATE WIND MOVEMENT. NOTICE MOST OF THE GAS MOVES TOWARDS THE NORTHWEST REGIONS OF THE MAP, WHY?
TRANSPORT of chemical compounds IN SOIL and groundwater
Soil is the lovely nourishing ground beneath us, it has a complex structure, and it’s structure is dependent on the chemical and biological composition of rocks + sand + clay + biotic material. Different soils have different compositions.
Chemicals can be deposited on top of soil (ex. oil spills, fertilizers, garbage). These chemicals move in soil by being ‘in solution’ aka dissolved in water (Aqueous).
Water movement in soil depends on how much ‘room’ it has to flow
PORES:
The tiny spaces between soil grains. Larger pores allow for groundwater to move quickly, smaller pores cause water to move slowly or not at all.
PERMEABLE SOIL:
Soil that contains interconnected pores which allows the quick movement of groundwater.
SANDY LOAM HAS MUCH LARGER PORES THAN CLAY LOAM
water moving through soil can dissolve and transport chemicals
LEACHATE:
ESPRESSO IS A LEACHATE - HURRAY FOR COFFEE!
A solution (usually water) that flows through a solid (like soil, or garbage) and dissolves some of its constituents (aka percolated)
Ex. Water + Coffee grinds = coffee solution (technically a leachate)
Ex. Water + Garbage pile = garbage water solution (technically a leachate)
Soils with a lot of organic material slows the movement of chemicals if they are absorbed by the organic material.
Clay prevents leachate’s movement
Sand increases leachate’s movement
When water percolates far enough it will reach a zone of soil called groundwater, this is soil saturated with water.
WATER TABLE:
The top of the groundwater zone. The groundwater zone contains no air spaces.
Groundwater moves in all directions (sideways, up, down) and can move very slowly (1m per year) or very quickly (1m per day). Composition of the soil affects the rate of the leachate’s movement
SITE A - WOULD LOOK LIKE THIS
SITE B - WOULD LOOK LIKE THIS
MANY LAYERS OF DIFFERENT SOIL TYPES
Solution movement in soil:
Evaporates/Residue - water evaporates out of chemical solution leaving chemical compound on top of the soil as a residue.
Collection - chemical solution flows over top of soil eventually collecting in nearby streams, rivers, lakes, and ocean
Absorption into plants - chemical solution soaks into the soil, it is absorbed by plants via its roots, possibly harming/burning plants
Absorption into soil - chemical solution is absorbed into the soil rather than plants, chemical solution may sit within the pores of the soil, or will continue to move downward with water, eventually pooling into ground water.
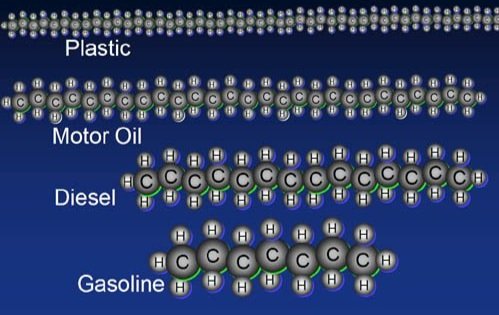
An example of chemical transport in soil: HYDROCARBONS
Hydrocarbons are carried by water in the soil
Hydrocarbons that do not dissolve coat grains of soil and fills up the pores in the soil, thus, concentration increases and also cleanup is difficult
Hydrocarbons are toxic to plants and animals (humans too!)
Natural Gas:
A fossil fuel that is gaseous/liquid found in the Earth’s crust, most of natural gas is methane.
Sour Gas - Natural gas that contains hydrogen sulfide (stinky!)
Sweet Gas - Natural gas that lacks hydrogen sulfide
Transport of chemical compounds IN SURFACE WATER
When a chemical substance is dumped into any body of water it’s considered to be waterborne. The chemical will scatter.
Waterborne chemicals will scatter in the water, how far they scatter depends on a few factors:
1. Source - chemical substances can enter surface waters from the air, groundwater, runoff, or outflow from sewers/sewage treatment plants.
2. Chemical’s characteristics - weight, size, surface area of chemical compound
3. Solubility - how well a chemical substance dissolves in water
4. Currents - direction and speed of water flow, ocean currents (ex. gulf stream) or river currents spread chemicals far, ground water moves slowly.
Remember S-C-S-C
CHEMICAL COMPOUNDS IN SURFACE WATERS
The Exxon Valdez Oil spill showcases how hazardous chemicals affect environments and the organisms living in those ecosystems.
chemical cycling - the Nitrogen Cycle
There are many different natural cycles that occur (water cycle, carbon cycle, oxygen cycle). Let’s take a look at a cycle that is crucial to life and growth of plants and animals on Earth - the nitrogen cycle.
THE NITROGEN IN THE AIR WE BREATH IS DIATOMIC, TWO NITROGENS SHARE 3 ELECTRONS
Every organism uses nitrogen to some extent. It’s found in plant and animal tissues. It even comprises about 78% of the air we breath.
However there is a catch: we can’t use atmospheric nitrogen!
This atmospheric nitrogen diatomic gas (N₂) is ‘free’ to use but it’s very non-reactive. The bond between diatomic nitrogen is very strong making N₂ chemically non-reactive. How does nature ‘fix’ this problem?
NODULES IN THE ROOT SYSTEM ALLOW FOR FREE NITROGEN TO BE FIXED SO THAT IT CAN BE USED BY PLANTS
CROSS SECTION OF A NODULE - BACTERIA LIVE IN TINY SPHERE LIKE ROOMS CREATED FOR THEM BY THE NODULES, MUCH LIKE A HOTEL
BACTERIA CAN ALSO FIX NITROGEN OUTSIDE OF THE ROOT NODULE JUST BY HANGING AROUND IN THE SOIL
Nitrogen Fixation:
Splitting nitrogen (N₂) into single usable nitrogen atoms. The single nitrogen combines with hydrogen to form the molecular compound of ammonia (NH₃).
Bacteria within the soil use the free nitrogen N₂ to make ammonia (NH₃)
Nitrify:
Converting molecules of ammonia NH₃ into polyatomic ions nitrite NO₂⁻ and nitrate NO₃⁻
Bacteria within the soil and attached to roots in legumes will further convert ammonia into nitrite NO₂⁻ and nitrate NO₃⁻ (plant food)
Note: there are other bacteria that can reverse the nitrifying reaction back into free nitrogen N₂, they denitrify it!
Nitrogen Cycle:
Free nitrogen → Ammonia → Nitrite & Nitrate (plant food) → Free nitrogen (via de-nitrifying bacteria)
Neat fact: lightning also adds nitrogen to soil, it zaps the atmospheric ‘free’ nitrogen to make NO₂
Fritz Haber
FRITZ HABER
Creator of the Haber process in 1910. Whereby air, methane, and water are used to create ammonia (NH₃). This invention alone has increased the yield of food stocks, if you are alive and eating there’s a good chance it’s due to this man!
Additionally, Fritz also invented the use of chlorine gas as a chemical weapon, which was used in the first world war. Chlorine gas binds with the mucous of your lungs to create Hydrochloric Acid.
The Haber process is still used to this day for agriculture (50% of the nitrogen in your body comes from this process). Chemical weapons not so much (Geneva Protocol).
How to lose nitrogen in soil:
De-nitrifying bacteria convert nitrate back into free nitrogen
Nitrates and nitrites are washed off the soil, or washed down deep where roots do not reach.
Farming - plants uptake nitrogen from soil, grow, then are harvested and eaten.
Nitrogen supplementation
aka Agricultural Activities
Before the invention of the Haber process usable ammonia came from natural sources such as manure and guano!
Fertilizer :
A BAG OF FERTILIZER SHOWING THE PERCENTAGES OF EACH NUTRIENT
A substance that enriches soil so that plants will grow larger and greener. Bags of fertilizer will show three numbers in this order: N-P-K. Also known as the NPK ratio it stands for percent nitrogen, phosphorous, potassium.
10 - 20 - 10
5 - 10 - 0
Why do the percentages not add up to 100% ? The rest is carrier material (peat soil, charcoal, mud)
The surface Run-off Effect
Fertilizers are washed away by rain creating a N-P rich solution that enters bodies of water
This temporary bump in nutrients increases the growth of algae and green plants in the water.
Without constant supply of nutrients, the party is over for the extra algae and plants
This dead organic matter is now food for bacteria, using up more dissolved oxygen in water.
Oxygen levels decrease in the water, causing dead zones, fish and aquatic insects asphyxiate (not enough oxygen)
Agricultural Pesticides
Pesticides :
General term for chemicals used to kill pests. Pests are organisms (plant or animal) that harm people, crops or structures. There are three general types:
Herbicides kill or control weeds.
Insecticides kill or control insects.
Fungicides kill fungi.
Farmers must be careful with the amount of pesticides circulated into the environment, there are a lot of unintended consequences.
Remember B-PACK!
B - Bioaccumulation of pesticide in animal food chains
P - Pesticide-resistance can occur pests that harm environments
A - Accumulate in the environment for a long time
C - Combines with other pesticides to form a more toxic chemical
K - Kills non-pests unintentionally
Biomagnification:
Is the increase in concentration of a chemical or element as it moves up the food chain. (Remember bioaccumulation refers to the accumulation of toxic substance in animal over time, ex. a polar bear accumulates more toxins as it eats seals over time)
For example, if algae are infected with mercury, insects eat many algae, each obtaining amounts of mercury; fish eat many of the infected insects therefore obtaining even more amounts of mercury; and this continues up the food chain.
How organisms uptake chemical compounds
Plants and animals rely on their environment to source the chemicals they need to build, maintain, and repair their structures.
Substrate:
A physical substance that an organism lives on, some organisms live off their substrate
Ex. Trees live on soil, fungus lives on decaying matter, barnacles live on a whale, moss on a rock
How Plants Uptake chemical compounds
Chemical Absorption:
In plants chemicals are absorbed through a membrane, there are two forms of absorption:
Passive transport
Active transport
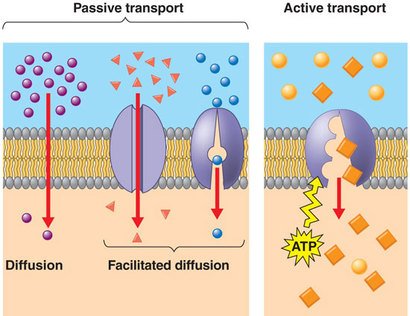
Passive Transport:
Compounds/nutrients move into plant cell without energy. Compounds just ‘pass’ through, hence passive transport. Two kinds of passive transport: diffusion and osmosis.
Diffusion:
CELL MEMBRANE VIEW OF CHEMICAL TRANSPORT THROUGH THE CELL MEMBRANE
Molecules move from an area of high solute concentration to an area of low solute concentration. (Particles even out or balance so the concentration of substances is equal)
Osmosis:
Water molecules move from an area of low solute concentration to an area of high solute concentration through a semi-permeable membrane. (Water moves towards salty solutions)
Active Transport:
When plant cells use energy to transporting compounds/nutrients from areas of low solute concentration to areas of high solute concentration.
how Animals Uptake chemical Compounds
Ingestion:
Consumption of a substance by an organism
Organisms break down food mechanically and chemically (using enzymes) through a
gastro-intestinal tract (tract means ‘passage’ btw).
Hydrolysis:
The reaction of a substance with water. ‘Hydro’ refers to water and ‘lysis’ refers to breakdown.
A substance has been hydrolyzed when it has been broken down by hydrolysis.
Salivary amylase speeds up hydrolysis of starch
How Fungi uptake chemical compounds
Most fungi release enzymes to breakdown dead material. This breaks down the material and makes the nutrients available for the fungi to absorb. Rather than have a stomach, fungi secrete a sort of gastric juice onto decaying matter, they digest the food on the outside of their bodies!
What are Nutrients?
EAT YOUR VEGGIES KIDS!
Chemical compounds that are absorbed or ingested by organisms are called nutrients. When an organisms takes up a chemical we call that chemical a nutrient.
Nutrient Compound:
Any element or chemical compound that an organism uses for living, growing and reproducing.
Macronutrients - chemicals that organisms need in large amounts
Ex. plants need Carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium
Ex. humans need Carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, magnesium, calcium and sulfur.
Micronutrients - chemicals that organisms need in small or trace amounts
Ex. humans need very small amounts of selenium, iron, iodine, copper, zinc, and all vitamins.
Organic compounds:
Molecules that contain carbon (usually bonded to hydrogen, but not always)
Named organic because these compounds were derived from living things (ex. C₆H₁₂O₆)
Vitamins are considered organic compounds (ex. vitamin K - C₃₁H₄₆O₂)
Inorganic compounds:
Molecules that do not contain carbon.
Named inorganic because these compounds were derived from minerals (ex. table salt - NaCl, calcium in milk - CaHPO₄)
There are many more organic than inorganic compounds on Earth!
Types of organic compounds
Four common groups of biological molecules:
Carbohydrates
Lipids (AKA fats)
Proteins
Nucleic Acids
IT’S NOT.
1. CARBOHYDRATES
Carbohydrates (Think fuel):
Organic macromolecule made up of carbon, hydrogen and oxygen. Three sub categories of carbohydrates.
Simple carbohydrates (generally known as sugars). Scientifically known as monosaccharides, a few examples:
Glucose (C₆H₁₂O₆) - product of photosynthesis
Fructose (C₆H₁₂O₆) - found in fruits
Galactose (C₆H₁₂O₆) - found in milk
When two simple carbohydrates link together they can form disaccharides:
Sucrose (C₁₂H₂₂O₁₁) - table sugar (glucose and fructose)
Maltose - soft drinks & candy (two glucoses)
Lactose - milk (glucose and galactose)
Complex carbohydrates (generally shortened to carbs). Scientifically known as polysaccharides, a few examples:
Starches (C₆H₁₀O₅)ᴺ - Plants store excess glucose as starch (humans can digest)
Cellulose (C₆H₁₀O₅)ᴺ - Plants build structures from glucose, such as cell walls and biofilms (humans can’t digest, but good bacteria loves it!)
Glycogen - Animals store extra glucose in their muscles and liver as glycogen. Depending on the animal only a small amount of glucose is stored as glycogen (600 g in humans). The rest is stored as fat.
2. LIPIDS:
Lipid (Think fuel storage):
Organic molecules made up of many carbon, hydrogen and oxygen atoms. (C₅₅H₁₀₄0₆).
Many different lipids are created to store excess sugars. This excess energy is stored as lipids, also known as ‘fats’. In humans if we have maxed out our glycogen storage (600 g) we then store sugars as 'fat’.
Plants and animals use lipids to create fats, oils and waxes for structural functions:
Blubber in animals
Oil on human skin
Waxes in a sperm whales head
Cell membranes are also mostly lipids (phospholipid bi-layer).
Fats/Lipids are broken down in the small intestine by bile which is shot in from the gall bladder. (Look up whale bile, it’s gross, and expensive)
3. PROTEINS
Protein (Think building blocks):
Very large organic compounds made up nitrogen, hydrogen, oxygen and carbon, sometimes sulfur.
AMINO ACIDS:
The building blocks of proteins, there are 20 different kinds, our body digests and up takes proteins in the form of amino acids.
Our body will build it’s own proteins from amino acids.
THE ‘TRAIN ENGINE’ OF PROTEINS. THE TRAIN CARS HOOK UP TO THE ‘R’ IN THE PROTEIN
Proteins follow a general structure much like a train. The structure is like a train engine followed by a number of train cars. There are 20 different kinds of ‘train cars’, and they can be added to the ‘train’ in different order to make different ‘trains’. Some amino acids trains can be up to 500 units long!
Our genes code for all proteins, proteins are created in the cell
Proteins make muscle fibres, skin, hair, nails, cellular structures, pretty much everything in an animal
Proteins make enzymes (catalyst of the body, ex amylase breaks down starch within seconds)
Proteins make hormones (hormones are the messengers of the body)
Proteins are broken down in the stomach by gastric juice and protease.
ENZYMES:
Special proteins that act as catalysts that control chemical reactions in organisms (Salivary Amylase, Protease)
4. NUCLEIC ACIDS:
NUcleic Acid (think instruction booklet):
The largest and most complicated molecules found in living things made mostly of nitrogen, nucleic acids play a major role in heredity and cell’s activities.
All cells contain 2 important nucleic acids:
Deoxyribonucleic acid (DNA) - a cell’s ‘instruction booklet’ on how to make different proteins
Ribonucleic acid (RNA) - a cell’s ‘post it note’ for holding copies of instructions from DNA
Broken down in the small intestine by deoxyribonuclease.
TESTING FOR ORGANIC MOLECULES
There are different tests to use when testing for different organic molecules.
GLUCOSE:
Benedict’s solution - turns from blue to yellow-orange-red in the presence of organic molecules.
STARCH:
Iodine solution - turns from red-brown to blue-black.
FAT/OIL:
Fats and oils - leave a spot on brown paper that light can pass through.
PROTEIN:
Biuret solution - turns from blue to purple to mauve.